Health
NAFDAC Raises Alarm Over ‘Toxic’ Cough Syrups
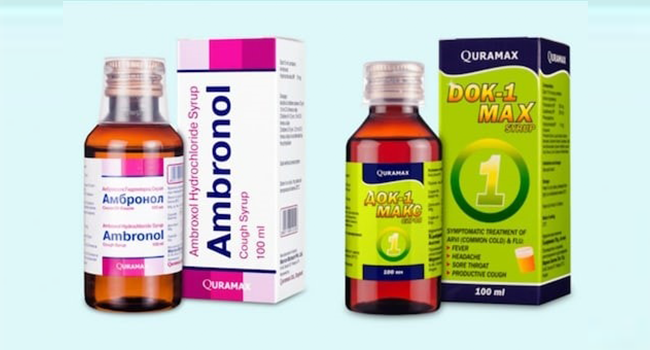
The National Agency for Food and Drug Administration and Control (NAFDAC) has called AMBRONOL and DOK-1 Max “infected” because they contain “toxic” chemicals.
In a statement posted on its website on Sunday, the group said that the items were found in the country of Uzbekistan in Central Asia and reported to the World Health Organization (WHO).
It called medical products that don’t meet quality standards “out of specification” because they don’t meet those standards.
According to NAFDAC, samples of both products were tested in the national quality control laboratories of the Ministry of Health of the Republic of Uzbekistan. The results showed that both products had too much diethylene glycol and/or ethylene glycol as impurities.
MarION Biotech Pvt. Ltd. is listed as the product’s maker for both items (Uttar Pradesh, India). The producer who is said to make these products has not yet given the WHO any guarantees that they are safe and reliable.
When taken by humans, diethylene glycol and ethylene glycol are fatally poisonous.
MarION Biotech Pvt. Ltd. is listed as the product’s manufacturer for both items (Uttar Pradesh, India). The producer who is said to make these products has not yet given the WHO any guarantees that they are safe and reliable.
When taken by humans, diethylene glycol and ethylene glycol are fatally poisonous. Abdominal discomfort, nausea, vomiting, diarrhea, the inability to pass urine, headaches, a changed mental status, and severe renal injury—which may result in death—can all have toxic consequences.
Because of their poor quality, these goods are dangerous, and using them, especially on children, could cause severe harm or even death.
Product Specifics
The specifics of the tainted (subpar) syrups are as follows:
The company that makes the product is called Marion Biotech Pvt. Ltd., and it is located in Uttar Pradesh, India.
Before using liquid dosage forms in medicines, manufacturers are urged by NAFDAC to conduct tests to determine whether contaminants like ethylene glycol and diethylene glycol are present. This is especially important for syrups with propylene glycol, polyethylene glycol, sorbitol, glycerin, or glycerol as fillers.
Even though the goods are not included in the NAFDAC database, importers, distributors, retailers, and customers are urged to exercise caution and vigilance throughout the supply chain to prevent the importation, distribution, sale, and consumption of the subpar (infected) syrups. All medical supplies must be purchased from approved and authorized vendors. Carefully examine the products’ physical integrity and genuineness.
People are asked to stop selling or using the items listed above and to bring any leftover stock to the NAFDAC office nearest them.
Please refrain from using these inferior products if you have them. You are encouraged to get immediate medical assistance from a licensed healthcare provider if you or someone you know has used these items or had any negative side effects.
The NAFDAC ADR e-Reporting Platform is accessible at www.nafdac.gov.ng. Healthcare professionals and consumers are encouraged to report adverse events or side effects related to the use of these products to the NAFDAC office that is closest to them, NAFDAC PRASCOR (20543 TOLL FREE from all networks), via pharmacovigilance@nafdac.gov.ng, or via email at pharmacovigilance@nafda.
NAFDAC is agency- and customer-oriented.
